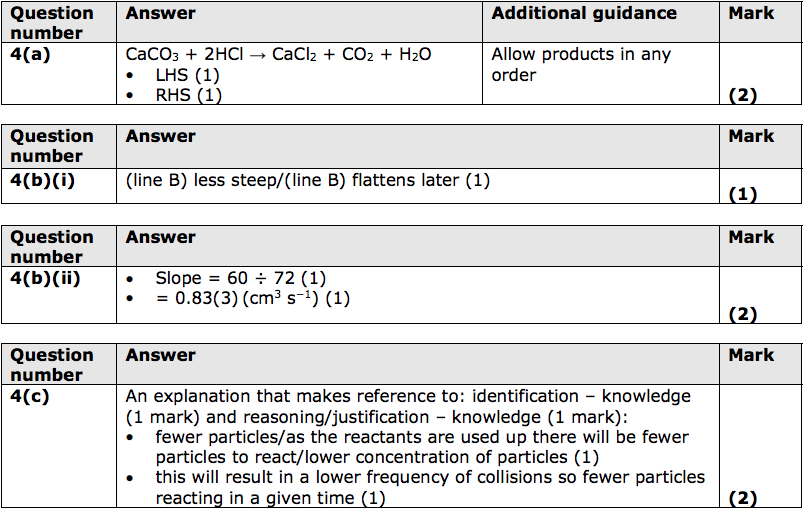
Caco3 2hcl cacl2 h2co3.
Marble and hydrochloric acid equation.
Calcium carbonate is dissolved by hydrochloric acid thereby forming gaseous carbon dioxide.
Hydrochloric acid 20ml 0 5m 1m 2m marble chips 2g per test large measuring cylinder plastic bowl 3 4 full of water rubber tubing glass conical flask stopwatch method.
D l2 aq h2o l co2 g the student used the apparatus shown in the.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
The rate of this reaction can be changed by changing the size of the marble chips.
The reaction takes place spontaneously.
Pieces of marble are thrown into hydro chloric acid.
Caco3 s 2hcl dt.
Balance the chemical equation for the reaction of calcium carbonate with hydrochloric acid.
Caco3 2hcl cacl2 h2o co2.
The student used an excess of marble.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
A strong effervescence can be observed.
The combined reactants have a higher chemical potential than the combined products i e.
The overall reaction is 2hcl caco3 cacl2 co2 h2o.
Calcium carbonate is dissolved by hydrochloric acid thereby forming gaseous carbon dioxide.
Caco3 hcl cacl2 co2 h2o to balance chemical equations we need to look at each element individually on both sides of the equation.
Marble is crystalized caco3.
The combined reactants have a higher chemical potential than the combined products i e.
Marble is mostly made up of calcium carbonate which is caco3.
Hydrochloric acid is hcl.
The reaction takes place spontaneously.
A student investigated the rate of reaction between marble and hydrochloric acid.
Acids carbonates salts carbon dioxide water marble is caco3 2hcl caco3 cacl2 co2 h2o.
This experiment is to show how much carbon dioxide is produced during the reaction between an acid hydrochloric acid and marble.
The reaction can be represented by this equation.
H2co3 decomposes easily into h2o and co2 so the equation is.