The reaction using a higher concentration of hydrochloric acid is faster.
Marble chips and hydrochloric acid observations.
Plot the results on the same graph.
Compare the slopes of the two graphs.
There are many variables that affect.
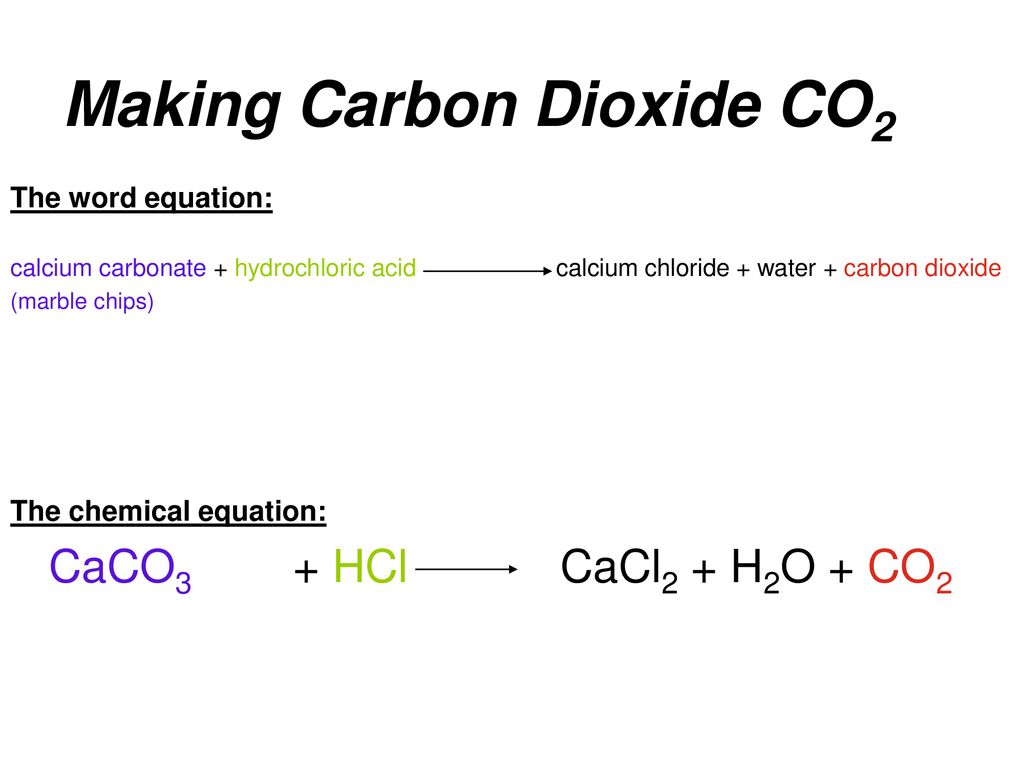
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
Finding the rate of reaction of marble chips and hydrochloric acid changing the surface area.
The rate of this reaction can be changed by changing the size of the marble chips.
A demonstration of the procedure for investigating the effect of concentration on the reaction between hydrochloric acid and calcium carbonate marble chips.