B marble calcium carbonate dissolves in hydrochloric acid to give calcium chloride water and carbon 16736972.
Marble dissolves in hcl.
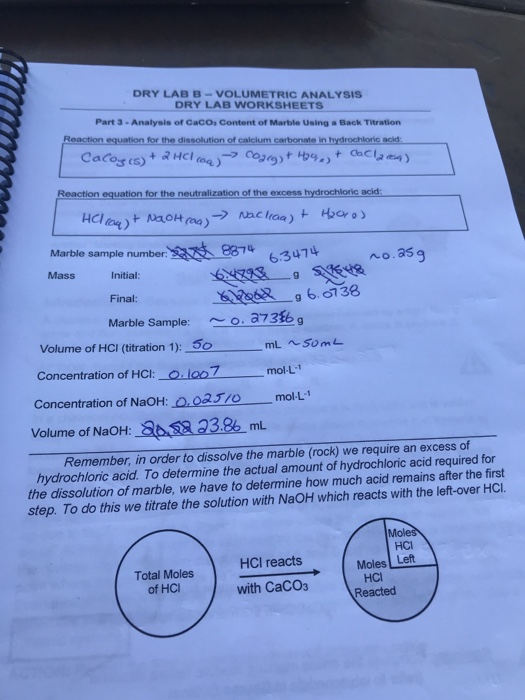
Caco3 2hcl cacl2 calcium chloride h2o water co2 carbon dioxide done.
The reaction takes place spontaneously.
The chemical equation for the reaction of marble with hydrochloric acid follows.
Limestone is composed almost entirely of calcite and will produce a vigorous fizz with a drop of hydrochloric acid.
I remember doing this one.
Dolostone is a rock composed of almost entirely of dolomite.
Marble chips and hydrochloric acid rates of reaction planning aim to find if changing the concentration of an acid will increase or decrease the rate of the reaction when marble is dissolved in hydrochloric acid.
1 mole of calcium carbonate reacts with 2 moles of hydrochloric acid to produce 1 mole of calcium chloride 1 mole of water and 1 mole of carbon dioxide.
I will weigh out one gram of marble chips using a balance and put it in a conical flask and add to it a concentration of 50cm3 using water and hydrochloric acid.
Being an alkaline substance and hydrochloric acid obviously acidic the two chemicals react.
Watch the resulting vigorous release of gas.
The chemical formula for the main composition of marble is.
The mass of the marble chips in each test tube.
A strong effervescence can be observed.
The combined reactants have a higher chemical potential than the combined products i e.
It will produce a very weak fizz when a drop of cold hydrochloric.
Dissolving calcium carbonate marble.
The acid test on rocks.
The time it takes for the marble chips to dissolve.
To ensure it is a fair test.
The different concentrations of diluted water mixed with hydrochloric acid.
By stoichiometry of the reaction.
Stone surface material may be lost all over or only in spots that are more reactive.
Marble is a crystalline form of calcium carbonate caco3 aka limestone.
When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves.
Some rocks contain carbonate minerals and the acid test can be used to help identify them.
The essay on marble chips hydrochloric acid.
Place a piece of calcium carbonate marble in a beaker and add a 10 hydrochloric acid solution.
The volume of the hydrochloric acid will need to stay the same.
Pieces of marble are thrown into hydro chloric acid.
Calcium carbonate is dissolved by hydrochloric acid thereby forming gaseous carbon dioxide.